Water exists in all the three states. When water gets freezes to 0 0 c degree Celsius it will turn to ice which is in solid form.
Ch104 Chapter 7 Solutions Chemistry
This can occur in two ways.

. Air for example is a solution. The simplest acid-base reactions are those of a strong acid with a strong base. They facilitate the formation of the transition state species within the reaction and speed up the rate of the reaction by a million-fold in comparison to non catalyzed reactions.
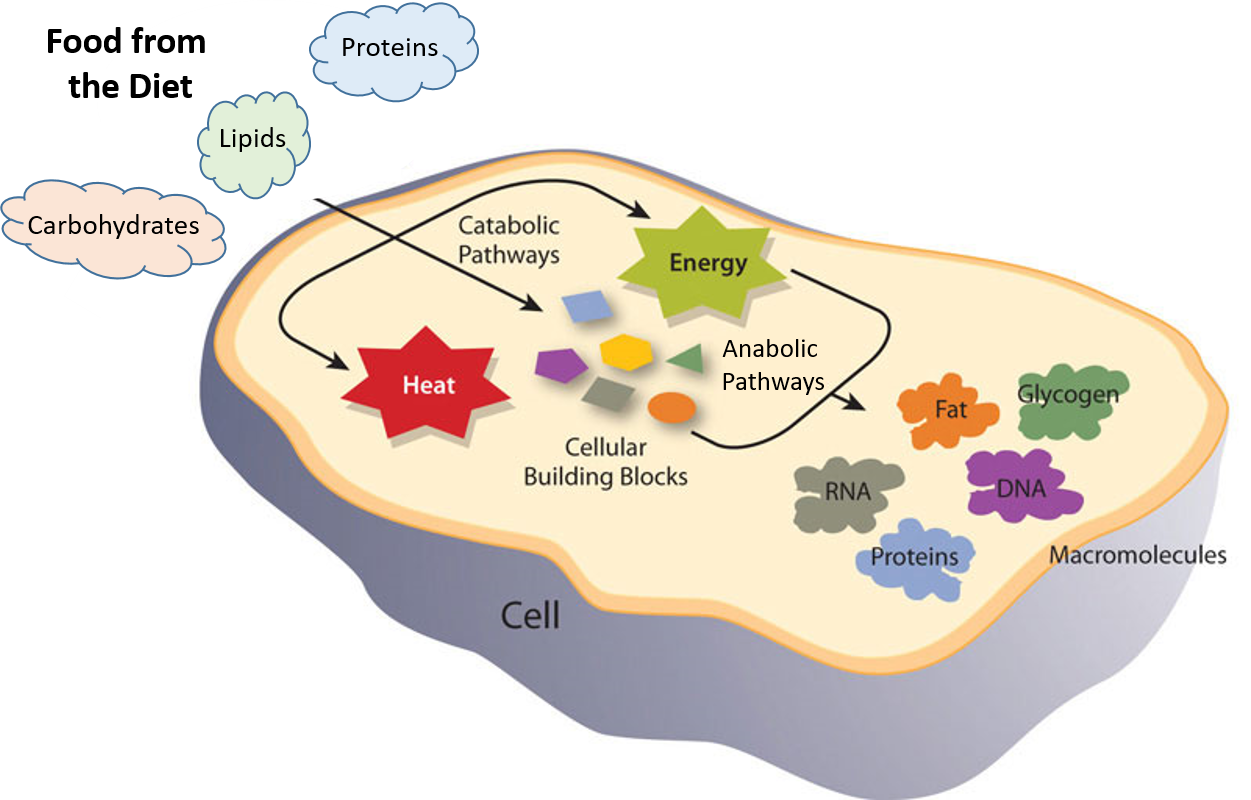
NCERT Solutions Class 11 Chemistry Chemistry Lab Manual Chemistry Sample Papers. An equation is balanced when the same number of each element is represented on the. Recall from Chapter 6 that enzymes are biological catalysts that reduce the activation energy required for a reaction to proceed in the forward direction Figure 71.
Answers to Chemistry End of Chapter Exercises. NCERT Solutions for Class 11 Chemistry Chapter 7 Equilibrium is provided on this page for the perusal of CBSE Class 11 Chemistry studentsDetailed step-by-step solutions for each and every intext and exercise question listed in Chapter 7 of the NCERT Class 11 Chemistry textbook categorized under the. We will learn more about the chemistry of soap-making in a later chapter section 124B.
Explain the formation of a chemical bond. Similarly the mass of 1 mol of helium atomic mass 4002602 amu is 4002602 g which is about one-third that of 1 mol of carbon-12. If section 46 were moved to chapter 12 then 56 and 75 would likely need to be moved into an organic or biological chemistry chapter as well.
B There is a net flow of water from the 4 starch solution into the 10 starch solution. It says something about the efficiency of natures designs. And animal cells Figure 74 An Idealized Animal Cell contain a cell nucleus that is also surrounded by a membrane and.
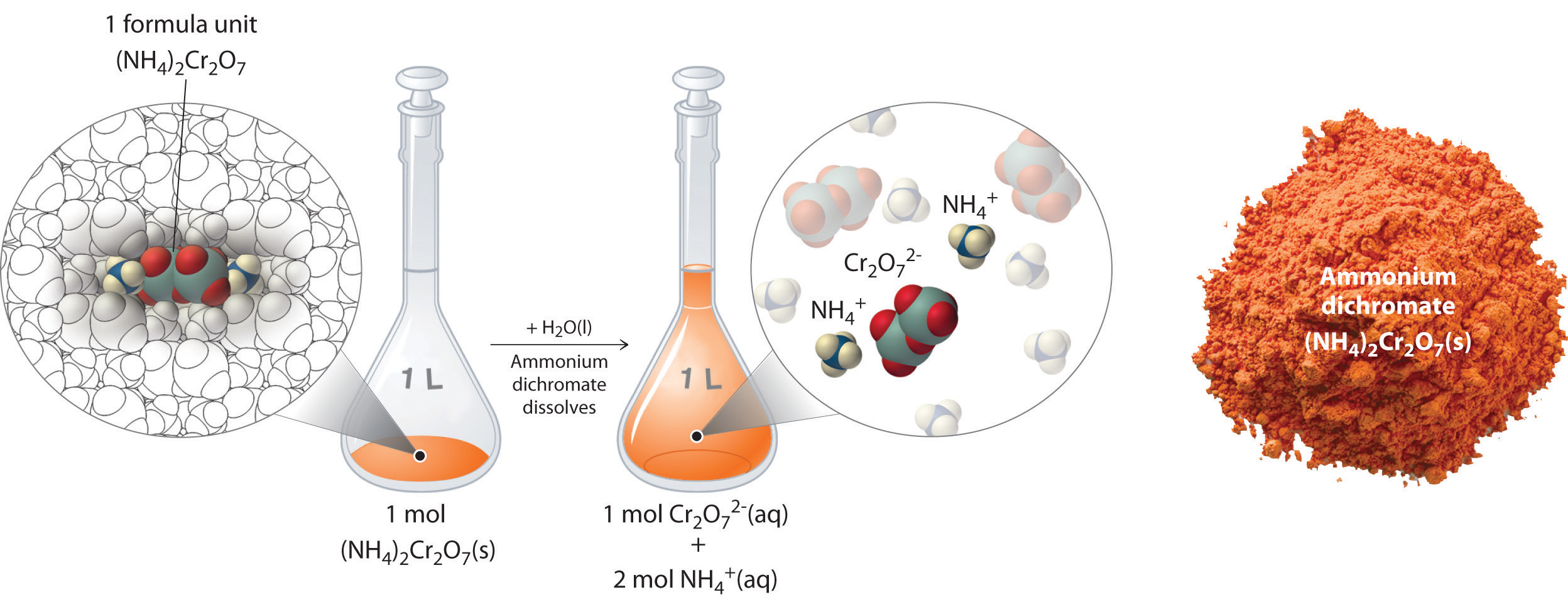
Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. A substance dissolved in a solvent to form a solution. Solutions are all around us.
E Starch moves out of the. Chapter 2 - Alcohols Phenols Thiols Ethers. Using the concept of the mole we can now restate Daltons theory.
Micelles will form spontaneously around small particles of oil that normally would not dissolve in water like that greasy spot on your shirt from the pepperoni slice that fell off your pizza and will carry the particle away with it into solution. If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution. Our bodies use carbohydrates primarily in the form of.
According to Kossel and Lewis atoms combine together in order to complete their respective octets so as to acquire the stable inert gas configuration. In free State water is a liquid. The values of the pH measured after successive additions of small amounts of NaOH are listed in the first column of this table and are graphed in Figure 1 in a form that is called a.
Table 4 shows data for the titration of a 250-mL sample of 0100 M hydrochloric acid with 0100 M sodium hydroxide. 1 mol of a compound is formed by combining elements in amounts whose mole ratios are small whole numbers. Chapter 1 - Organic Chemistry Review Hydrocarbons.
Access Answers of Selina publication ICSE Class 9 Chemistry Chapter 3 Water. Chemistry 101 - Chapter 4. 75x10-4 mol AgNO3 x 1 mol AG3PO4 3 mol AgNO3 25x10-4 mol K3PO4.
D Water does not cross the membrane at all. This is normally the component present in the smaller amount. NCERT TEXTBOOK QUESTIONS SOLVED.
When atoms gain or lose electrons to yield ions or combine with other atoms to form molecules their symbols are modified or combined to generate chemical formulas that appropriately represent these species. NCERT Solutions for Class 11 Chemistry Chapter 7 Free PDF Download. The other sections that could fit within either a general or organicbiological chemistry chapter are sections 56 redox in organic and biochemistry and 75 energy of biochemical reactions.
C There is a net flow of water from the 10 starch solution into the 4 starch solution.

Basic Balancing Chemical Equations Worksheet Template

Ncert Book Class 12 Chemistry Chapter 7 The P Block Elements

Selina Concise Chemistry Class 6 Icse Solutions Chapter 7 Water Learn Cram Learncram Selinaconcisechemistryclass6 Chemistry Class Study Chemistry Chemistry

Form 4 Notes Chapter 1 2 Potential Energy Physics Mechanics Chemistry Notes

Lakhmir Singh Science Class 8 Solutions For Chapter 14 Chemical Effect Of Electric Current Free Pdf

Selina Concise Chemistry Class 6 Icse Solutions Chapter 7 Water Cbse Tuts Cbsetuts Selinaconcis Chemistry Class Chemistry Book Pdf Chemistry Question Paper

Selina Solutions Class 9 Concise Chemistry Chapter 1 The Language Of Chemistry Download Free Pdf

Rd Sharma Solutions For Class 7 Maths Chapter 7 Algebraic Expressions Download Free Pdf

Release Chemistry Textbook Chemistry Molecular Geometry

Ncert Solutions For Class 12 Maths Exercise 7 7 Chapter 7 Integrals Free Pdf Download
Ch103 Chapter 7 Chemical Reactions In Biological Systems Chemistry

Lakhmir Singh Chemistry Class 9 Solutions For Chapter 2 Is Matter Around Us Pure Free Pdf

Selina Solutions Class 9 Concise Chemistry Chapter 1 The Language Of Chemistry Download Free Pdf

Rd Sharma Solutions For Class 7 Maths Chapter 7 Algebraic Expressions Download Free Pdf

Rd Sharma Solutions For Class 7 Maths Chapter 7 Algebraic Expressions Download Free Pdf

Plus Two Chemistry Notes Chapter 7 The P Block Elements A Plus Topper Https Www Aplustopper Com Plus Two Plus Two Chemistry Notes Chemistry Notes Chemistry

Rd Sharma Solutions For Class 7 Maths Chapter 6 Exponents Download Free Pdf

Rd Sharma Solutions For Class 7 Maths Chapter 7 Algebraic Expressions Download Free Pdf

Types Of Chemical Reactions Detailed Explanation With Example Videos

